Why do mosquitoes love me so much?
Ever thought you are more attractive to mosquitoes than your companions? Are you always the one covered in bites? There is a scientific reason why. Researchers in Kenya have been investigating what makes victims attractive to mosquitoes. Their studies identified four groups of chemical compounds in body odor that could be used to develop more effective bait traps for controlling dengue and chikungunya.
Female mosquitoes need to feed on human blood; it provides the protein and iron she needs for her eggs. Studies have shown mosquitoes are attracted to the CO2 in our breath, and to physical cues such as heat and moisture, along with visual stimuli such as light, color, and form.
While other studies have shown body odor also has a role to play, Dr. Eunice Anyango Owino, Medical Entomologist at the School of Biological Sciences, University of Nairobi, wanted to find out more: “We wanted to test the entire body odor, not just the breath.”
Mosquitoes love our body odor
In their first study, Dr. Owino and her team compared commercially available bait traps with bait traps made from natural body odors. They used samples of body odor trapped in used socks and worn t-shirts.
“When we compared the body odor traps to the commercial versions, we found the mosquitoes were more attracted to the body odor traps,” said Dr. Owino. “Once we established this, we then analyzed the body odor and identified the most potent chemical compounds.”
To extract the body odor from the clothing, volunteers working with the team wore the socks and t-shirts for 18 hours before placing them in tightly sealed collection jars. The odors were then absorbed into special fibers using suction provided by a vacuum.
Chemicals identified
An analytical method known as gas chromatography-mass spectrometry (GC-MS) identified the chemical components in body odor. Another method, known as gas chromatography-electroantennographic detection (GC-EAD), identified which specific chemicals mosquitoes can pick up using their antenna.
“We found the mosquitoes are attracted to four groups of compounds: aldehydes, fatty acids, ketones, and alcohols,” said Dr. Owino. “These four major groups of compounds are also the main chemical compounds that make a human odor. It’s an evolutionary relationship between mosquitoes and their human hosts.”
While our body odor mainly comes from aldehydes and fatty acids, the exact mix of these chemical compounds varies between individuals – which is why some people are more attractive to mosquitoes than others.
Read about Dengue and ethnicity and learn who is most at risk.
The mix also changes across different points of our bodies. “Certain parts of the body are more or less attractive to the mosquito,” said Dr. Owino.
Building better bait traps
For their second study, the team studied the effectiveness of these compounds by placing them into baited traps. They found combining the compounds with carbon dioxide sharpened mosquitoes’ ability to pick up body odors.
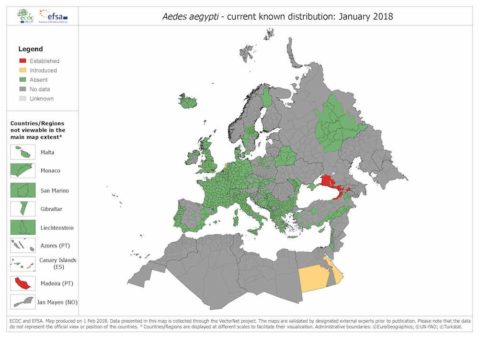
“Our results suggest there are additional chemical compounds that could potentially be commercialized to attract mosquitoes, particularly Aedes aegypti,” said Dr. Owino. “This could lead to a technology being made from compounds from human body odors to lure and kill mosquitoes that spread the dengue and chikungunya viruses.”
With chemical compound mix and release rate critical to how mosquitoes react, future work will focus on optimizing these. The team will also try to identify other compounds in body odor that could also attract mosquitoes. Once the team has identified these, they will need to test them to see if they are also capable of being used as bait for mosquitoes.
“We aim to come up with a super bait for mosquitoes to control transmission of the dengue and chikungunya viruses,” said Dr. Owino. “I believe in a good bait that we can produce at a low price.”
—
Are you an expert on dengue? Click here to join Dengue Lab and contribute to the discussion around this disease.