- by Alison
Dengue fever vaccine pipeline update: the latest progress on protection against the virus

The dengue fever vaccine pipeline is advancing and as candidates approach clinical trials, we decided to take a closer look at the vaccines that could ease the burden of this disease.
The world’s first dengue vaccine, Dengvaxia, was licensed by Sanofi Pasteur in 2015. It is available in more than a dozen countries and can prevent all four serotypes of dengue fever. (See the World Health Organisation’s Q&A on the dengue vaccine.)
While Dengvaxia was the first vaccine against dengue fever, there are several others at various stages of development. We last looked at the dengue vaccine pipeline back in April of 2017.
Since that time, progress has continued to develop at a steady pace. Amongst other things, Takeda and the WRAIR, GSK & Fiocruz collaboration have recently published data; Takeda has also initiated a second phase III trial, and Panacea Biotec is expecting to start a clinical trial sometime later this year.
Let’s examine the status of the dengue vaccine candidates currently in or approaching clinical trials – all of which, incidentally, target all four dengue serotypes:
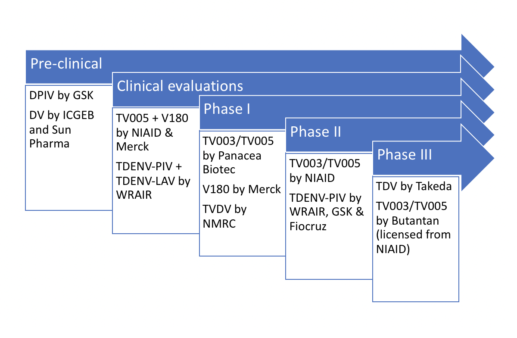
Phase III trials
TDV
Takeda’s TDV dengue vaccine is a good place to start. Arguably the most advanced vaccine candidate currently in the pipeline, TDV is a live-attenuated and recombinant vaccine, meaning it is made up of a weakened live dengue virus and produced through recombinant DNA technology. It is intended to be given in two doses, 90 days apart.
Takeda began its phase III trials of TDV began back in September 2016. The first of these trials, a large 5-year study in Asia and Latin America, aims to test both how well the vaccine prevents dengue fever and the long-term side effects of the vaccine in children. It began a second phase III trial in December 2017. This 12-month study is testing TDV on 400 healthy adolescents in non-endemic areas in Mexico.
In addition, Takeda recently published the interim data from its ongoing phase II trial of TDV on children in Asia and Latin America.
According to the published results, TDV is safe and produces an immune response in children aged 2-17 years, irrespective of previous exposure to dengue – validating the initiation of its phase III trials.
TV003/TV005
Developed by the US-government funded NIAID (part of the United States National Institutes of Health (US NIH)), TV005 and TV003 are variations of the same live-attenuated and recombinant vaccine, except that the former has ten times more dengue serotype 2 component than the latter. Both are intended to be given as a single dose.
NIAID has licensed TV003/TV005 to several manufacturers for further development. These include Instituto Butantan in Brazil; VaBiotech in Vietnam; Panacea Biotec, Serum Institute of India and Indian Immunologicals in India; Medigen Biotech in Taiwan; and Merck.
In February 2016, Instituto Butantan began a large phase III community-based trial on both children and adults in urban areas with dengue transmission across Brazil.
Phase II trials
TV003/TV005
NIAID is also sponsoring a clinical trial of TV003/TV005 in countries such as Thailand and Bangladesh. The on-going phase II trial of TV003 in Thailand is expected to complete in August, while the phase II trial of TV005 in Bangladesh is not expected to complete until 2020.
According to First World Pharma, Panacea Biotec has secured permission to conduct phase I/II trials in India, with trials expected to start in 2018. Other manufacturers are still in the preclinical phase.
TDENV-PIV
The vaccine candidate TDENV-PIV emerged from a collaboration between GlaxoSmithKline (GSK), Walter Reed Army Institute of Research (WRAIR) and Fiocruz. TDENV-PIV is a purified inactivated vaccine, meaning it is made up of dead purified components of the dengue virus with an ‘adjuvant‘ (a substance that helps boost the body’s immune reaction). Like TDV, TDENV-PIV is given in two doses, in this case, four weeks apart.
Its on-going phase II trial on 140 adults in the U.S. is helping it further evaluate and refine TDENV-PIV. The collaboration recently published results from its phase I trial of TDENV-PIV in the U.S. but has not yet published results from its phase I trial of the vaccine in Puerto Rico
GSK is developing its DPIV vaccine candidate, from the knowledge gained from this collaboration. DPIV has not yet reached clinical trials.
Phase I trials
Merck’s V180 is a recombinant subunit dengue vaccine, meaning it only contains essential antigens and not all the other molecules that make up the virus. It is intended to be administered in three doses, one month apart. Merck’s phase I trials in the US and Australia tested different doses of the vaccine candidate in combination with different ‘adjuvants’.
Also in phase, I, the U.S. Naval Medical Research Center (NMRC) has evaluated its DNA vaccine (a technique using genetically engineered DNA to stimulate the body’s immune system) in combination with an adjuvant. While TVDV, as it is known, targets all four dengue serotypes, an earlier phase I trial evaluated its DENV-1 component, which is known as D1ME100.
Dengue fever vaccine pipeline: other evaluations
In addition to the clinical trials mentioned above, other trials are testing vaccines in combination to glean more information on how our bodies respond to dengue infection and vaccines, along with how the vaccines complement each other.
First, WRAIR is combining two dengue vaccines using a ‘prime-boost strategy’ that primes the body’s immune system with a first vaccine before boosting its immune response with a second. Its trial to test TDENV-PIV with an adjuvant in combination with TDENV-LAV was withdrawn in April 2017 because it wasn’t moving forward. TDENV-PIV is a purified inactivated vaccine while TDENV-LAV is a live attenuated vaccine. WRAIR is, however, currently recruiting for a new phase I study to test TDENV-PIV as a prime vaccine in combination with a TDENV-LAV booster. The study will compare different dosing schedules, where the booster is given 90 or 180 days after the main vaccine.
Second, a collaboration between NIAID and Merck has tested a combination of dengue vaccine candidates TV005 and V180 in a phase I trial using a prime-boost strategy.
Third, Takeda is expected to begin a phase III trial in February, testing TDV alongside a Yellow Fever vaccine known as YF-17D.
Pre-clinical developments
One final development worth mentioning, The Hindu has reported that a collaboration between The International Centre for Genetic Biotechnology (ICGEB) and Sun Pharma is also developing a dengue vaccine. DSV4, as it is known, is intended to be administered at in three doses, each a month apart. The collaboration is expected to begin phase I trials in 2020.
The dengue fever vaccine pipeline is continuing to make great steps forward, developing a variety of vaccines to help combat this terrible disease.
Additional sources:
http://www.who.int/research-observatory/monitoring/processes/health_products/en/
http://www.who.int/immunization/research/vaccine_pipeline_tracker_spreadsheet/en/
https://www.sciencedirect.com/science/article/pii/S0264410X17311647

