Aedes aegypti and Aedes albopictus are spreading across the globe, increasing the number of people at risk from mosquito-borne diseases, including dengue. Their expansion is largely due to human movement and climate change. Scientists are using statistical modelling techniques to show how Aedes is spreading. We asked them which regions the dengue vectors have populated today, where their model anticipates the mosquitoes will spread to next and how their predictions are helping slow – or even halt – the spread of Aedes.
“Aedes aegypti and Aedes albopictus are found in all continents except Antarctica,” revealed Dr Moritz Kraemer from the Department of Zoology at the University of Oxford. “Aedes aegypti is distributed around the tropics and subtropics. Aedes albopictus can also be found in temperate regions, especially in China, the US and Europe.”
Aedes spreading across Europe and the US
Despite the spread of Aedes expanding over the last 40 years, a lack of data means it’s often difficult to pinpoint their distribution during that period. That said, authorities in Europe and the US have been monitoring Aedes closely.
Dr Kraemer confirmed our own analysis of Europe’s emerging dengue threat by saying, “Over the past 10 to 15 years, Europe has seen a rapid expansion of Aedes albopictus northward towards more temperate regions, from Albania into Italy and from Italy towards France and over the Alps into Switzerland and Germany.” His viewpoint also aligns with the European Centre for Disease Prevention and Control (ECDC), which reports “Aedes albopictus is currently considered a serious biting nuisance for humans in Italy, southern France and Spain.”
The US is the only place where Aedes aegypti has been monitored well enough at a continental level, according to Dr Kraemer. Data from the late 1980s onwards shows how the primary dengue vector has spread along the highway network “coming from the southeastern US into more northern and western parts,” he says.
Predicting the spread of Aedes
The researchers are using mathematical modelling to predict how both Aedes species might spread in response to accelerating urbanisation, human movement and climate change. It is unique in that, unlike previous models, it takes the impact of human movement into account. “We are interested in how Aedes spreads to new places, looking at both the environment and who might import the vector,” says Dr Kraemer.
The mosquitoes can only fly around 100 metres (and on occasion a bit further), so they need to be transported to new regions. While anecdotal evidence shows they have arrived on cars or by laying their eggs in ships, this movement had not previously been quantified or used to predict future spread.
The model also predicts and takes into account future urbanisation processes, specifically focused around Asia and sub-Saharan Africa where vast areas will become urbanised. Dr Kraemer adds, “The movement of people into urban areas is very important when you’re looking specifically at Aedes aegypti. People are travelling from areas where there are mosquitoes and importing eggs or even adults into new locations.”
Urbanisation behind the spread of Aedes aegypti
Indeed, the model showed Aedes aegypti moving into urban areas because they are climactically suitable. “These mosquitoes breed in artificial containers near houses where humans live,” said Dr Kraemer. Urban areas potentially at risk include the high population centres in the coastal areas of China and the US, where we have already seen importations of the species.
In regions where the mosquito is already a problem – Brazil, Asia or sub-Saharan Africa in particular – the model doesn’t see such rapid expansion in mosquito populations or invasion to new areas. Rather, the model showed mosquitoes might be able to transmit disease for longer. “The current six months when the mosquito can transmit disease may extend to eight or nine months as temperatures increase,” says Dr Kraemer. That can have devastating impacts on disease transmission, he adds.
The model has also predicted that some areas of the US, such as the centre mid-west, are becoming too dry for the mosquito.
Aedes albopictus spreads opportunistically
The model predicts Aedes albopictus to expand along the fringes of its current population in a “wave-like” fashion. “We anticipate the niche Aedes albopictus can occupy today will be filled over the next ten to fifteen years,” says Dr Kraemer. “We expect it to spread to habitats where it can survive but isn’t present today, then to new habitats where increasing temperatures or increasing precipitation make the environment suitable for these mosquitoes to establish new populations.”
Targetted surveillance and control
Global surveillance and control efforts that aim to mitigate the spread of vector-borne diseases such as dengue need to understand how Aedes is spreading. Experts in the ECDC in Europe and US Centre for Disease Control (CDC) can use the model’s maps and predictions to identify locations most at risk of Aedes invasion so they can target surveillance and control programmes more effectively.
“We’re not just thinking about where the mosquito might arrive but where Aedes might spread and become established,” says Dr Kraemer. “If we can prevent it from establishing its population in a new place, that would help slow down its spread.”
The model’s European maps show regions in northern France, Belgium, the Netherlands and northern parts of Germany at risk. Dr Kraemer explains how having pinpointed those locations; “collaborators set mosquito traps along highways or in other places where the mosquitoes are likely to arrive. If they find mosquitoes, they can start preventing those populations from growing and establishing themselves in those places.”
Improving predictions
The team feeds the information from the local surveys in Europe back into their model to update their model predictions to become more relevant to the local context. They do the same with the information gleaned from their surveys in the US, Indonesia and Brazil. The model’s massive dataset of up to 40,000 records for both species also collates data from literature and government agencies.
Going forward, the team would also like to include data from social media and citizen science approaches. “We are looking at ways of validating data from people on the ground who could feed information back to us in a more real-time fashion,” says Dr Kraemer. “People who have been bitten by a mosquito might send a picture of the mosquito so an expert can identify it. That would be a very great opportunity to advance the quality and reliability of our model.”
Following their interest in monitoring where new introductions are coming from, the team are also looking at using mosquitoes’ genetic code to relate Aedes populations within their model. “It would be interesting to see whether new Aedes populations coming into California originate from central America or the eastern US,” says Dr Kraemer.
“Genetic sequencing has already been used for tracing Zika virus from the Americas, yellow fever throughout Brazil, and the importation of chikungunya into the Americas from Angola. It helps us to stratify the geographic landscape of disease. If we could do that for mosquitoes, that would improve the accuracy of our methodologies,” he concludes.
We can’t wait to see the model evolve in the coming months and years. Get in contact with us if you are working on your own models to predict the spread of Aedes.


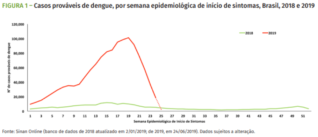
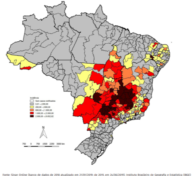 Dengue is suspected of striking down almost two per cent of the inhabitants of Minas Gerais, that’s one in every 50 people. Next hardest hit is Goiás in the mid-west where the virus has infected 1.3 per cent of inhabitants. Located in the western limits of the northern region of Brazil, the rural state of Acre
Dengue is suspected of striking down almost two per cent of the inhabitants of Minas Gerais, that’s one in every 50 people. Next hardest hit is Goiás in the mid-west where the virus has infected 1.3 per cent of inhabitants. Located in the western limits of the northern region of Brazil, the rural state of Acre