- by Alison
The dengue vaccine pipeline: what’s next?
 The dengue vaccine pipeline continues to develop steadily. We last took a look at the state of dengue vaccine development back in October 2014. Since then, Sanofi Pasteur’s CYD-TDV has been licensed, trademarked as Dengvaxia®, and two vaccine candidates – one developed by Takeda and another by the National Institute of Allergy and Infectious Diseases (NIAID) and Instituto Butantan – have entered phase III trials.
The dengue vaccine pipeline continues to develop steadily. We last took a look at the state of dengue vaccine development back in October 2014. Since then, Sanofi Pasteur’s CYD-TDV has been licensed, trademarked as Dengvaxia®, and two vaccine candidates – one developed by Takeda and another by the National Institute of Allergy and Infectious Diseases (NIAID) and Instituto Butantan – have entered phase III trials.
We spoke with In-Kyu Yoon, Deputy Director General of Science at the International Vaccine Institute (IVI) and Director of the Global Dengue + Aedes-Transmitted Diseases Consortium (GDAC), to learn more about where the dengue vaccine pipeline stands tod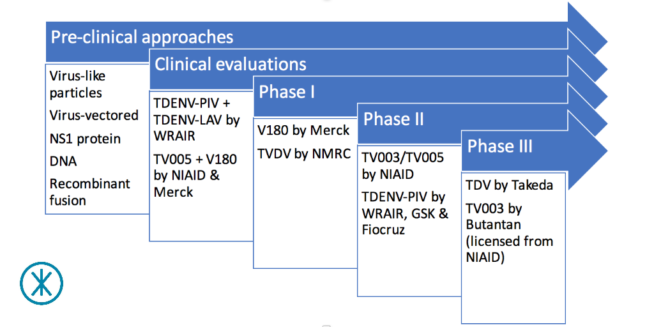 ay.
ay.
The dengue vaccine pipeline in 2017
Phase III trials
Formerly known as DENVax, Takeda’s TDV (or TAK-003) is given in two doses 90 days apart and targets all four dengue serotypes. Takeda has completed phase II trials in Puerto Rico, Colombia, Singapore and Thailand to test the safety and immune response of the candidate vaccine. These were followed by further phase II trials in the Dominican Republic, Panama, and the Philippines to refine the dosing. A phase III trial, aimed at testing its ability to provide the desired protection, began in Asia and Latin America last September.
Developed by the US-government funded NIAID (part of the United States National Institutes of Health (US NIH)), TV003/TV005 also targets all four dengue serotypes, but, unlike TDV, is intended to be given as a single dose. TV005 and TV003 are variations of the same vaccine except that the former has 10 times more dengue serotype 2 component than the latter. NIAID has licensed TV003/TV005 to several manufacturers for further development. These include Instituto Butantan in Brazil; VaBiotech in Vietnam; Panacea Biotec, Serum Institute of India and Indian Immunologicals in India; Medigen Biotech in Taiwan; and Merck. Instituto Butantan began a large phase III trial across Brazil in February 2016, while the other manufacturers are still in the preclinical stage.
Phase II trials
NIAID has also sponsored phase II trials of TV003/TV005 in countries such as Thailand and Bangladesh.
The vaccine candidate TDENV-PIV emerged from a collaboration between GlaxoSmithKline (GSK), Walter Reed Army Institute of Research (WRAIR) and Fiocruz. TDENV-PIV is a purified inactivated vaccine (meaning it is made up of dead purified components of the dengue virus). From the knowledge gained from this collaboration, GSK is developing its DPIV vaccine candidate, which has not yet reached clinical trials. Like TDV, TDENV-PIV is given in two doses, which are four weeks – as opposed to 90 days – apart. Having completed phase I trials in the US and Puerto Rico, the collaboration is currently evaluating and refining TDENV-PIV in a phase II trial in the US.
Phase I trials
Merck’s V180 was again designed to protect against all four dengue serotypes. Phase I trials in the US and Australia tested different doses of the vaccine candidate in combination with different ‘adjuvants’ – substances that help to boost the body’s immune reaction.
Also in phase I, TVDV was developed by the U.S. Naval Medical Research Center (NMRC) and has been evaluated in combination with an adjuvant in a phase I trial. While TVDV targets all four dengue serotypes, an earlier phase I trial evaluated its DENV-1 component, which is known as D1ME100.
Other evaluations
In addition to the clinical trials mentioned above, other trials are testing vaccines in combination to glean more information on how our bodies respond to dengue infection and vaccines, along with how the vaccines complement each other. The vaccines in the examples below are combined using a ‘prime-boost strategy’, which primes the body’s immune system with a first vaccine before boosting its immune response with a second.
Firstly, WRAIR is testing TDENV-PIV with an adjuvant in combination with TDENV-LAV. TDENV-PIV is a purified inactivated vaccine while TDENV-LAV is a live attenuated vaccine (meaning it is made up of a weakened live dengue virus).
Secondly, a collaboration between NIAID and Merck is testing a combination of the vaccine candidates TV005 and V180 in a phase II trial.
Pre-clinical developments
Before we finish, it’s worth noting that there a lot of other dengue vaccine approaches currently in pre-clinical development. Three, in particular, are probably worth a quick mention. The first approach is based on dengue virus-like particles (or VLPs). These vaccine candidates use structures that look similar to dengue virus to induce an immune response. The second approach is based on virus vectors. These vaccine candidates use a modified virus to carry parts of the dengue vaccine to stimulate an immune response.
The third approach focuses on the non-structural protein-1 (NS1 protein), a distinct protein within the dengue virus that researchers believe may play a fundamental role in bringing about vascular leakage in people with severe dengue. These vaccine candidates aim to induce an immune response that protects against the potentially lethal DENV-induced vascular leakage.
It’s heartening to see that the dengue vaccine pipeline continues to evolve, making great steps forward in developing a variety of vaccines to combat this terrible disease.
Could a shift in vaccine confidence towards other vaccines affect dengue vaccination rates?
—
Create a record of dengue fever near you. Click below to report Aedes activity to Dengue Track.

