- by Gary Finnegan
EU green-lights first vaccine for prevention of dengue
The decision focuses on overseas territories – but climate change could bring mosquito-borne diseases to northern Europe
The European Medicines Agency – the EU drug watchdog – has paved the way for the approval of the first vaccine for prevention of dengue. The EMA’s influential Committee for Medical Products for Human Use (CHMP) recommended that Dengvaxia be granted a marketing authorization. The European Commission has the final say but this is usually a formality for medicines and vaccines given the green light by key EMA experts.
‘Dengue is by far the most common mosquito-borne viral disease affecting people worldwide…’
‘Dengue is by far the most common mosquito-borne viral disease affecting people worldwide (mainly in tropical areas); tens of millions of cases occur each year resulting in approximately 20,000-25,000 deaths, mainly in children,’ the EMA said, announcing its decision.
The regulator said that while most people affected by one of the four strains of dengue virus recover well, around 2% suffer severe dengue which can be lethal. There is currently no specific treatment for dengue and, until the development of the first vaccine against dengue, prevention has been limited to the environmental management of mosquitoes.
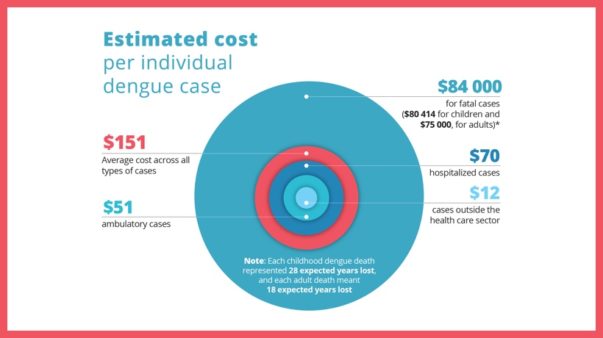
The dengue vaccine recommendation excludes the EU mainland and territories outside tropical areas. ‘A number of EU territories, mainly overseas, are situated in endemic areas, and these territories could benefit from this vaccine,’ the regulator said.
Dengue in Europe is nothing new. In fact, outbreaks of the disease have been recorded on the continent for almost a century, but most cases have been reported in tropical outposts such as Reunion Island and Madeira. However, both Spain and France recorded locally transmitted dengue cases in 2018. And climate modeling research suggests that global warming could make mainland Europe more hospitable to mosquitoes, raising the risk of dengue outbreaks to northern cities including Berlin and Stockholm.
Shot in the arm
The decision is a shot in the arm for the vaccine which had faced political challenges in the Philippines. The first public dengue vaccination program was introduced by a Manila government, only for its successor to suspend the initiative.
The controversy in the Philippines stems from the public and political response to data released by the vaccine’s manufacturer, Sanofi Pasteur, in late 2017 showing that the vaccine is best suited to people who have had prior dengue infections. This is because a second dengue infection can be worse than a first infection and the body responds to the vaccine as though it were the first infection. As a result, the WHO updated its advice on the vaccine, recommending that it be used in endemic areas and for people who have had already been exposed to the dengue virus.
The EMA’s CHMP also recommended limiting the use of the vaccine to individuals with prior dengue virus infection, for whom laboratory confirmation of the previous infection is available before vaccination. ‘In people who have never had dengue, there is an increased risk of clinically severe dengue disease leading to hospitalization when vaccinees are subsequently infected with dengue virus,’ it said.
‘In addition, because there are no safety, immunogenicity or efficacy data to support the vaccination of individuals living in non-endemic areas and traveling to endemic areas, vaccination of these individuals is not recommended.’

In their decision to recommend approval of the vaccine, EMA experts pointed to 31 clinical studies in dengue-endemic areas, mostly in Latin America and the Asia Pacific. These trials included over 41,000 participants aged 9 months to 60 years receiving at least one dose of the vaccine.
‘The overall available data demonstrate that for people between 9 and 45 years of age, the vaccine has positive effects in preventing symptomatic and severe dengue disease in people who have had previous dengue infection and live in endemic areas.’
Dengvaxia, the first vaccine for the prevention of dengue fever. Several other dengue vaccines, currently in the dengue vaccine pipeline are expected to be available in the years to come.
—
Help create an international day focused on dengue fever and the prevention of this disease. Sign our joint petition for a World Dengue Day, below.

